Building Earth's Elements out of Clay
Creating clay molecules
In these geology activities you will create clay molecules of water, sodium, carbon and silica. You will be creating three molecules that are the building blocks of life on our planet. You will be creating a water molecule, salt molecule, and a carbon dioxide.
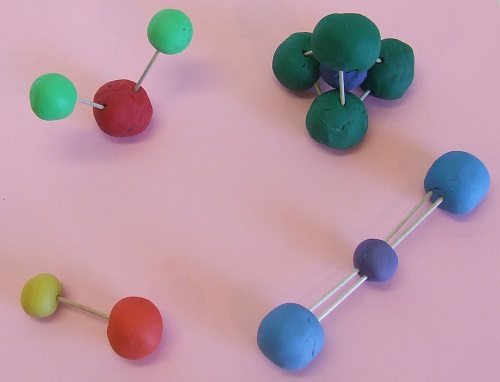
Silica is the most abundant mineral and it forms its crystal structure as a tetrahedron shown in the upper right-hand corner of the picture. Each of these molecules are essential for life to exist on Earth.
Materials
- Different colors of clay
- Toothpicks
Directions
Water molecule -
- Create one 2-inch ball of clay for the oxygen
atom and two 1- inch balls of clay for the hydrogen atoms.
- Attach the two smaller balls with a single toothpick to the oxygen atom. The three atoms form a water molecule.
Halite - Salt
- Create a 2-inch ball for the sodium chloride atom
and a 2.5 inch ball of another color for the chlorine molecule.
- Combine the two atoms together with a single toothpick to form a halite molecule.
Carbon dioxide
- Create two 2-inch oxygen atoms and one 1.5-inch
carbon atom.
- Place the carbon atom in the center of the other two atoms.
- Attach the three atoms together to form a carbon dioxide molecule by using two toothpicks on each side of the carbon atom.
Silica tetrahedron
- Create four 2-inch oxygen atoms and one 1-inch
silica atom.
- Form an equilateral triangle base by joining three oxygen
atoms together with toothpicks.
- Place the silica atom in the center of
the triangle.
- Place a toothpick on the top of each of the oxygen
atoms so them come together at a point.
- Press the fourth oxygen atom into the three toothpicks forming your silica tetrahedron.

Our Rocks & Mineral activity books contains 48 pages of fun activities about our planet. Activities include Creating Sugar Cube Caves, Colorful Rock Trivet and a Crystal Salt Garden. Myrna Martin

Click for More Information and to Order
Science behind the activity
Water is a universal solvent. It dissolves most materials found on Earth including rocks exposed on its surface due to erosion. Oil is an example of molecules that are repelled by water and do not dissolve in it.
Halite is common mineral that we know as salt. We use it to salt our food and it is the same salt found in the oceans. chemists call the mineral sodium chloride.
Carbon dioxide When we breathe in air it contains a mixture of gases including oxygen. When we exhale the air out of our lungs the main gas is carbon dixoide. Carbon dioxide in the air is used by plants for photosynthesis. A by-product of photosynthesis is the oxygen we breathe.
The silica tetrahedron has a negative charge so it bonds with many compounds by sharing oxygen atoms to form 95% of the minerals found on the Earth's surface that is why it was included in these geology activities.
KIDS FUN Science Bookstore
Check out Myrna Martin's award winning textbooks, e-books, videos and rock sets. The Kids Fun Science Bookstore covers a wide range of earth science topics. Click here to browse.










